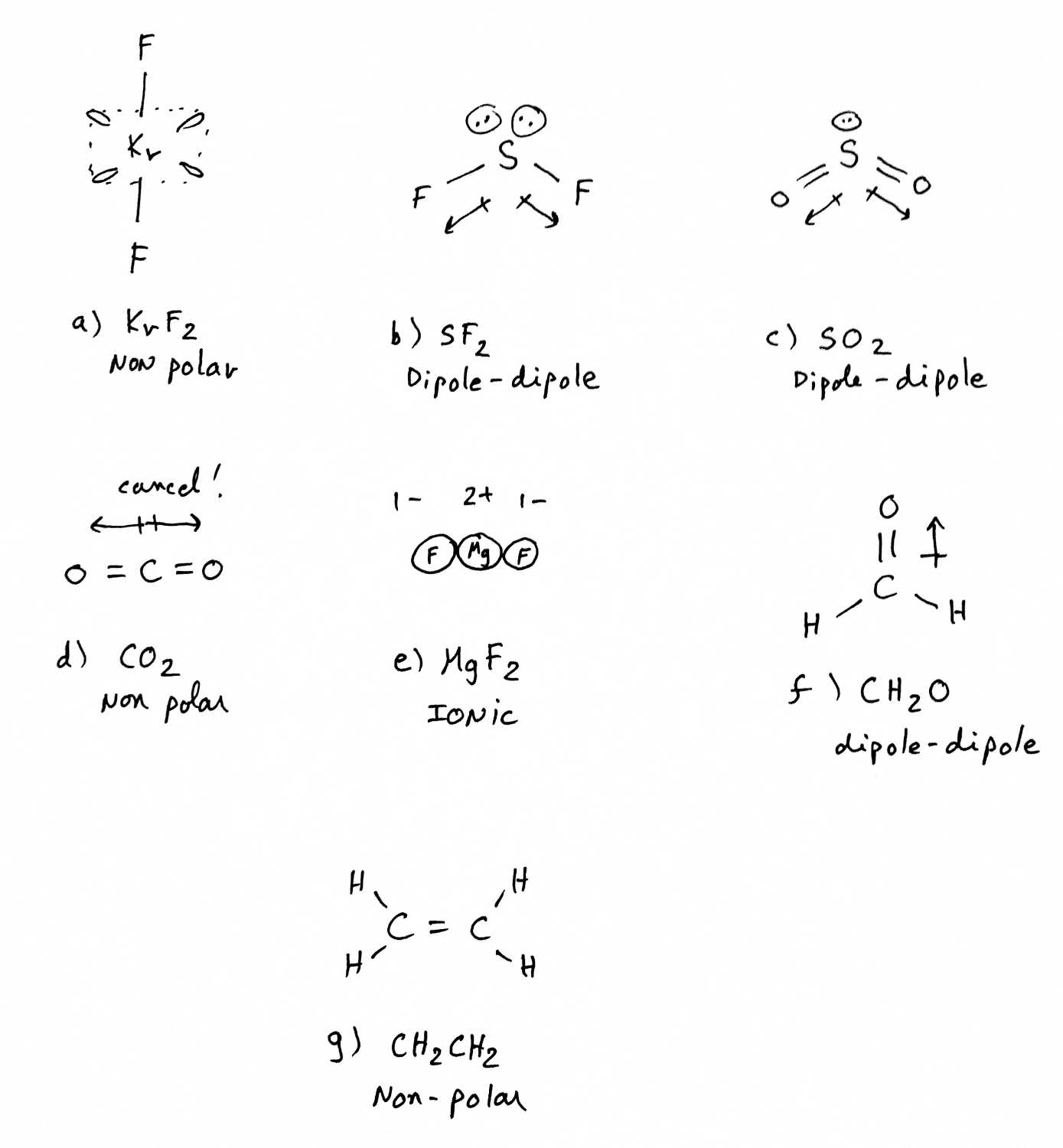
Problem 11-63 |
"Likes dissolve likes"
water: dissolve ionic compounds and any compound capable of creating H-bond or dipole-dipole interaction with water.
Carbon tetrachloride, despite the presence of Cl atoms, is a non polar solvent (tetrahedral = no dipole moment).
Therefore, it dissolves non-polar compounds or those that can make “London dispersion forces”.
This problem is actually a revision of the Lewis structure-VSEPR concept.