|
|
| DATA and INFORMATION |
| Ion concentration in the water |
| ion | concentration mg / L sea water |
| Cl- | 224000 |
| Mg2+ | 44000 |
| Na+ | 40100 |
| Ca2+ | 17200 |
| K+ | 7650 |
| Br- | 5300 |
|
|
Other information |
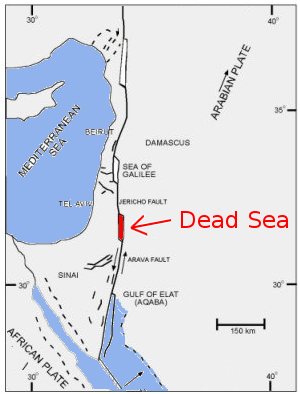
- Lowest place on earth: - 480 m vs. ocean level.
- Evaporation of the lake's waters: 1,4 m a year (estimated)
- The salt concentration is 10 time higher than the one of the ocean
- The density of the water is 1.24 g/mL
- Water temperature at the lowest level of the Dead Sea: 22 °C (constant)
|
|
Assumption |
No ion pairing (which is probably wrong a those concentrations!)
|
| Sources |
Dead Sea research center,
www.britannica.com ,
http:wikipedia.org ,
www.nasa.gov,
|
| SOLUTION |
The freezing point depression equation will be use for this problem
The total concentrations of all the ions (in molal: mol solute /kg solvent) has to be calculated
The following sample calculations are for the chloride ion. The calculation is the same for all the other ions |
| mass of Cl- in 1 L solution |
| Mass of Cl- = |
224000 mg |
| 1 L |
|
| Mass of 1 L of solution |
| 1 L solution = 1000. mL X |
1.24 g |
| 1 mL |
|
Total mass of all the ions in 1240 g solution
|
| ion | concentration (mg / L sea water) | mass (g) |
| Cl- | 224000 | 224 |
| Mg2+ | 44000 | 44.0 |
| Na+ | 40100 | 40.1 |
| Ca2+ | 17200 | 17.2 |
| K+ | 7650 | 7.65 |
| Br- | 5300 | 5.30 |
| Total | 338 |
|
| Mass of solvent in 1 L solution |
mass water (solvent) = 1240 g solution - 338 g solute (total) = 902 g solvent (0.902 kg solvent)
|
| Mole of Cl- in 1 L solution |
| Mole of Cl- = 224 g X |
1 mol |
| 35.45 g |
|
| Molality of Cl- |
|
Total concentration of particles
|
| ion | concentration (mg / L sea water) | concentration (molal) |
| Cl- | 224000 | 7.01 |
| Mg2+ | 44000 | 2.01 |
| Na+ | 40100 | 1.93 |
| Ca2+ | 17200 | 0.476 |
| K+ | 7650 | 0.217 |
| Br- | 5300 | 0.074 |
| Total molality of particles (ions) | 11.7 |
|
| Freezing point depression |
Tf (pure water) = 0.00 °C
Kf (water) = 1.86 °C.kg.mol-1
ΔTf = i Kf (solvent) m
Note: here, m = molality of all the particles, then the van't Hoff factor ( i ) = 1.
ΔTf = Kf (water) m
Finally: ΔTf = 1.86 °C.kg.mol-1 x 11.7 mol.kg-1 = 21.8 °C
Since the freezing point of pure water = 0 °C,then, the freezing point of the Dead sea = -21.8 °C
|
| ANSWER: -21.8 °C
(Actual value -21.1 °C) ,
|