The atomic theory (Zumdahl, Chapter 7.1 to 7.5)
(For this section you need to use the equations in section "Atomic structure and Periodicity")
The electromagnetic Spectrum
The Rydberg equation

The Rydberg equation
Photon (description, energy and calculations)

The Photon

Energy of the photon (calculations)
At his point, you should be able to do the following problems Zumdahl 11th ed: Chap 7. # 51, 53, 57, 59, 62, 159.
The Bohr's model of the atom

The Bohr model of the atom. Note: 1eV = 1.6022x10-19 J
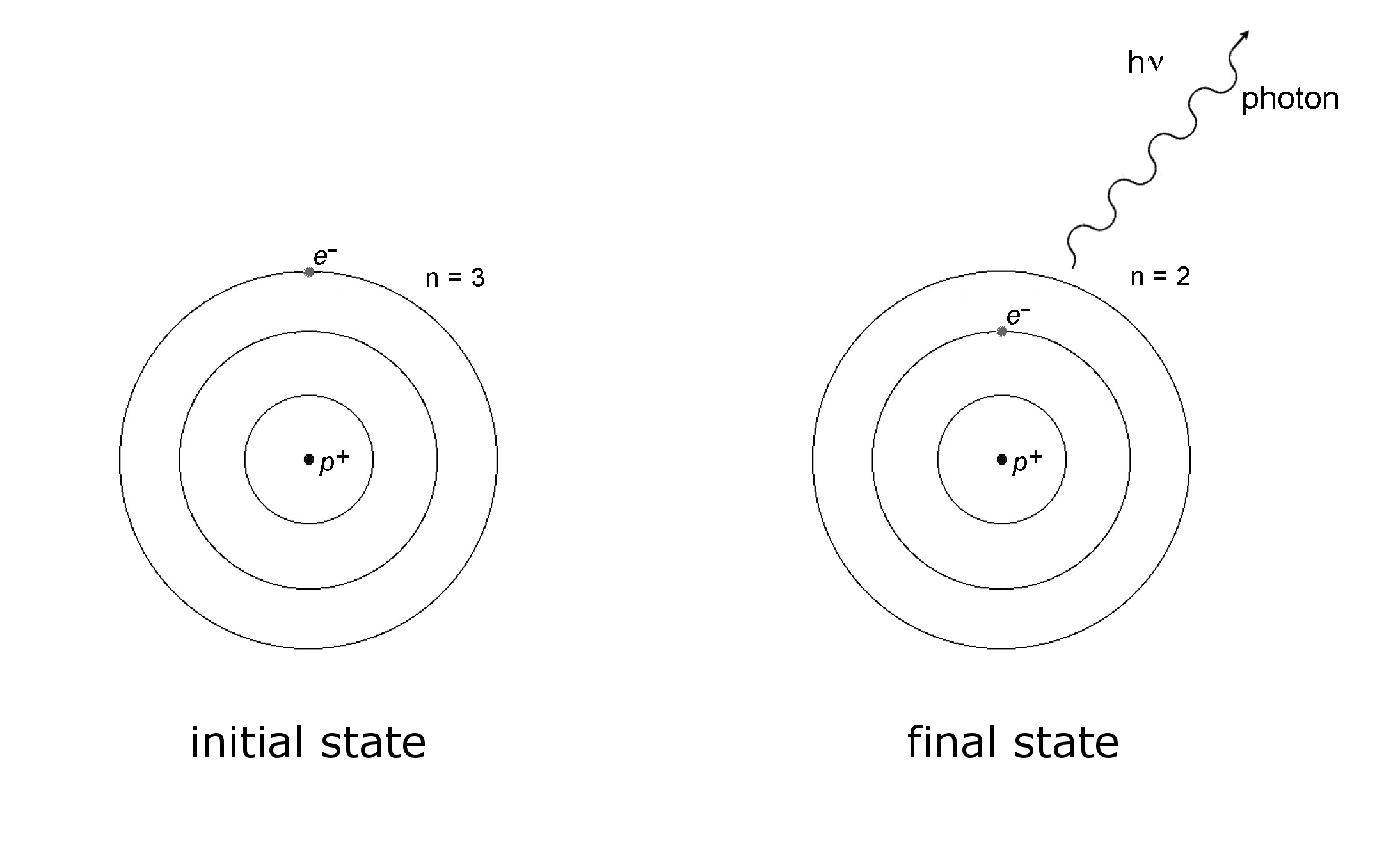
A simple and direct explanation of the model (with Video)

An history of the model
The corresponding problems to this section are: Zumdahl 11th ed: Chap 7. # 69, 71, 73, 78, 186.
Louis de Broglie's explanation of Bohr's atomic model

Electrons behave like a wave
Sample calculations