Periodic trends
Using the orbitals to predict the chemical behavior of the elements.
First: Some definitions
All physical and chemical behavior of the elements are based on the electron configuration of their atoms
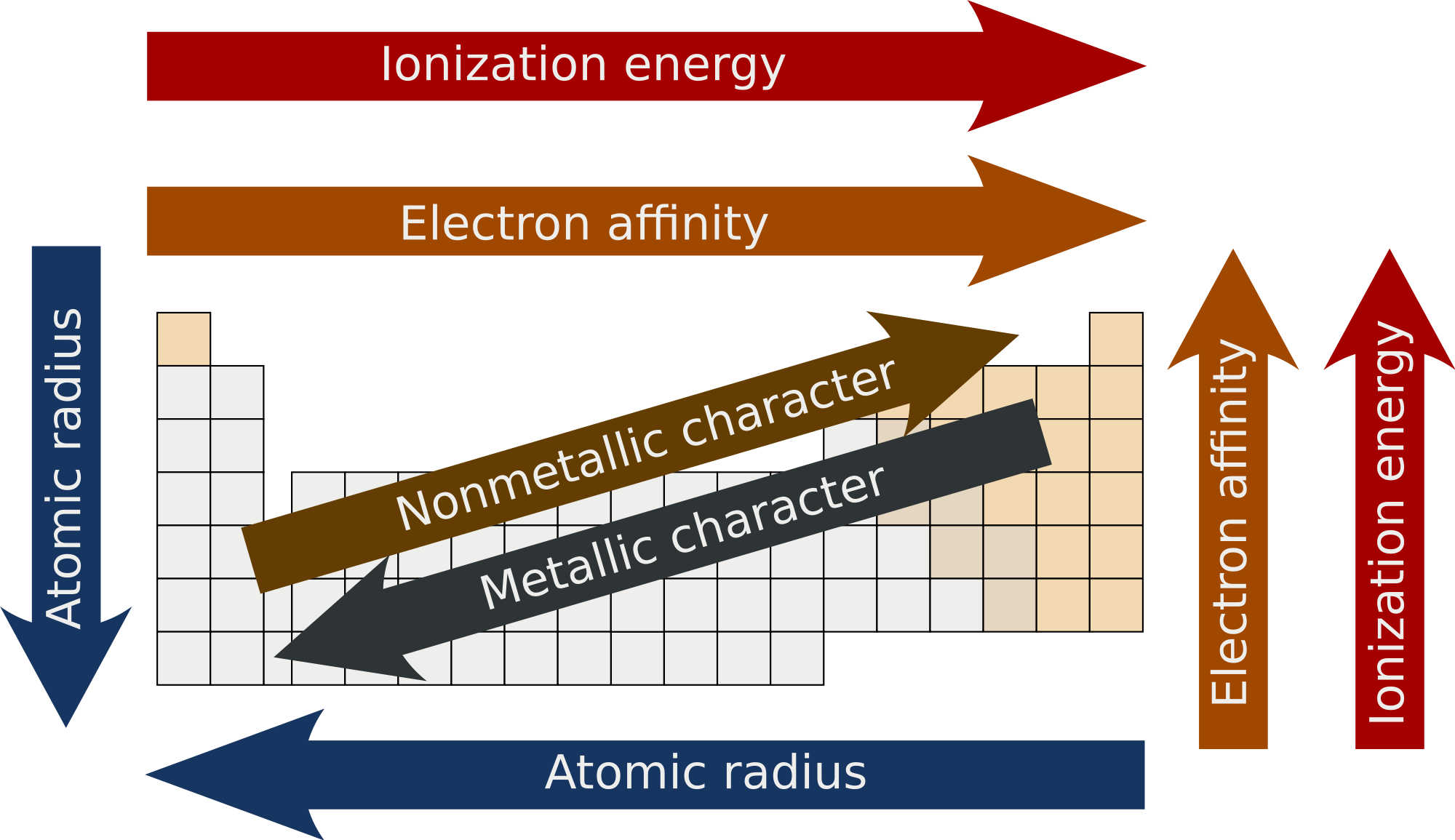
- Atomic radius: the size of an atom.
- Ionic radius: The size of an ion (anion = negative, cation = positive).
- Ionization energy (In): The energy required to remove an electron from an atom (to become a cation).
- Electron affinity (Eea): the energy associated to the gain of an electron (to become an anion).
- Electronegativity (EN): the potential of an atom to attract electrons in a covalent bond.
The atomic radius (or size)
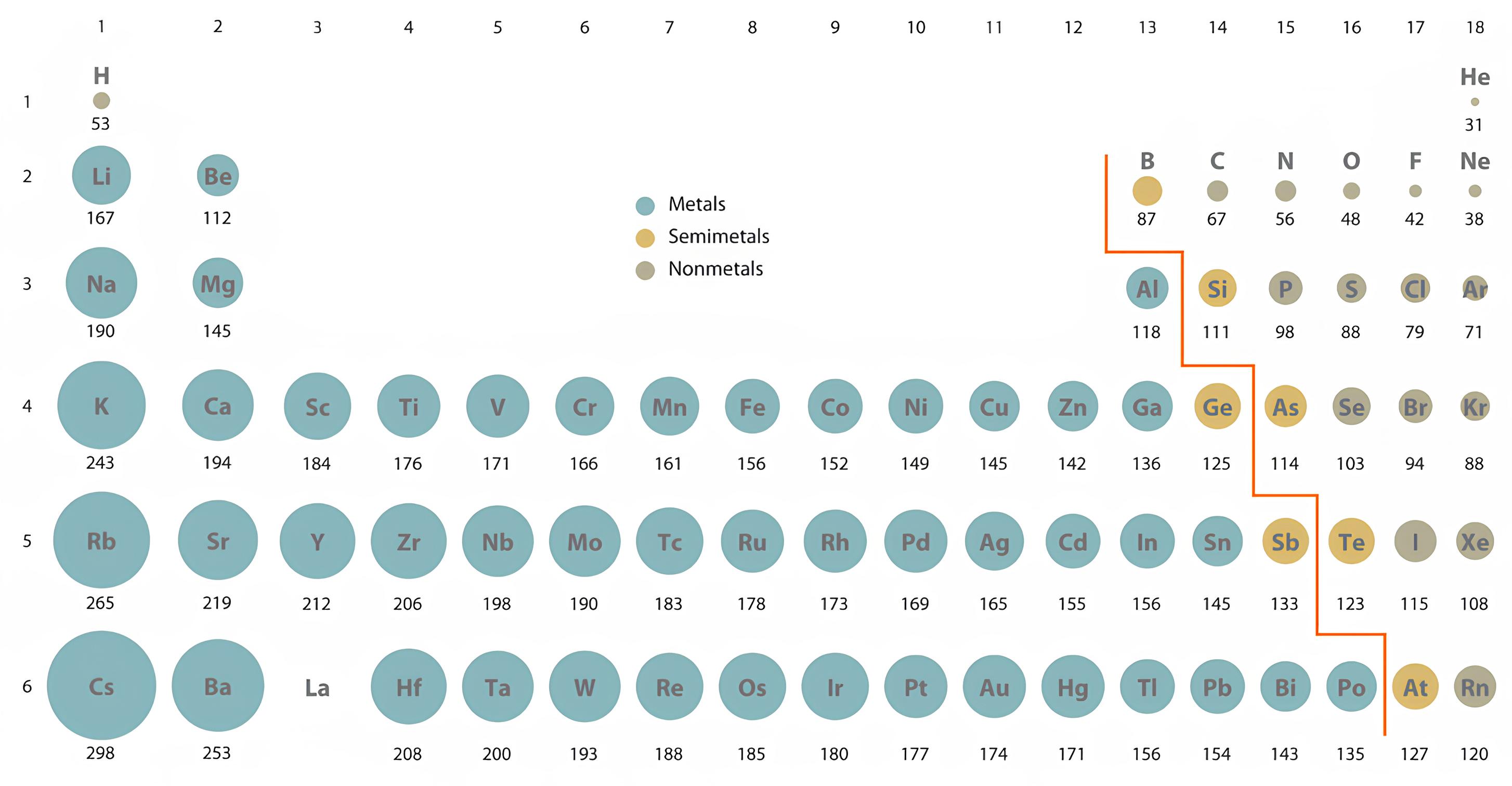
The size increases down a period since electron shells are added (higher orbital value n).
It is one of the most important trends of the periodic table size can also explain other chemical behaviors.
The ionic radius
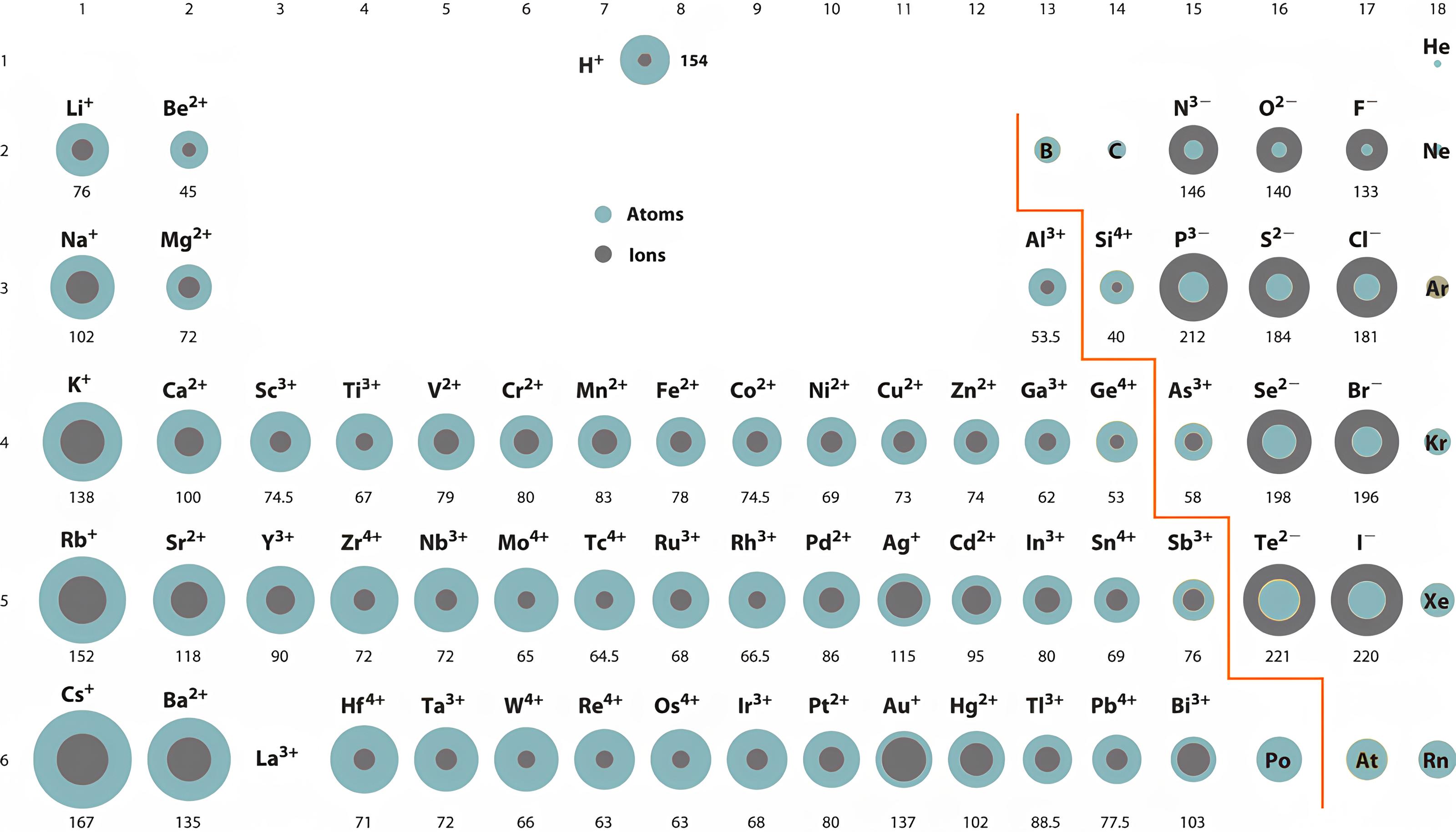
The size of an ion increases when the atom has gained an electron
Cations (or ions +) are small because there are more protons than electrons, therefore, electrons are more attracted to the nucleus.
Anion (or ion-) are larger because there are more electrons than protons. Additional electrons are less attracted to the nucleus.
Isoelectronic series
The set of ions below is called an isoelectronic series. They all have the same number of electrons (electronic configuration: 1s22s22p6). However, they have a different number of protons. In this case, the one with the largest number of protons (here Mg2+) will also be the smallest one since it is the ion with the largest attraction forces for the electrons. Consequently, the one with the fewest protons ( O2-), will also have the largest size (more electronic repulsion).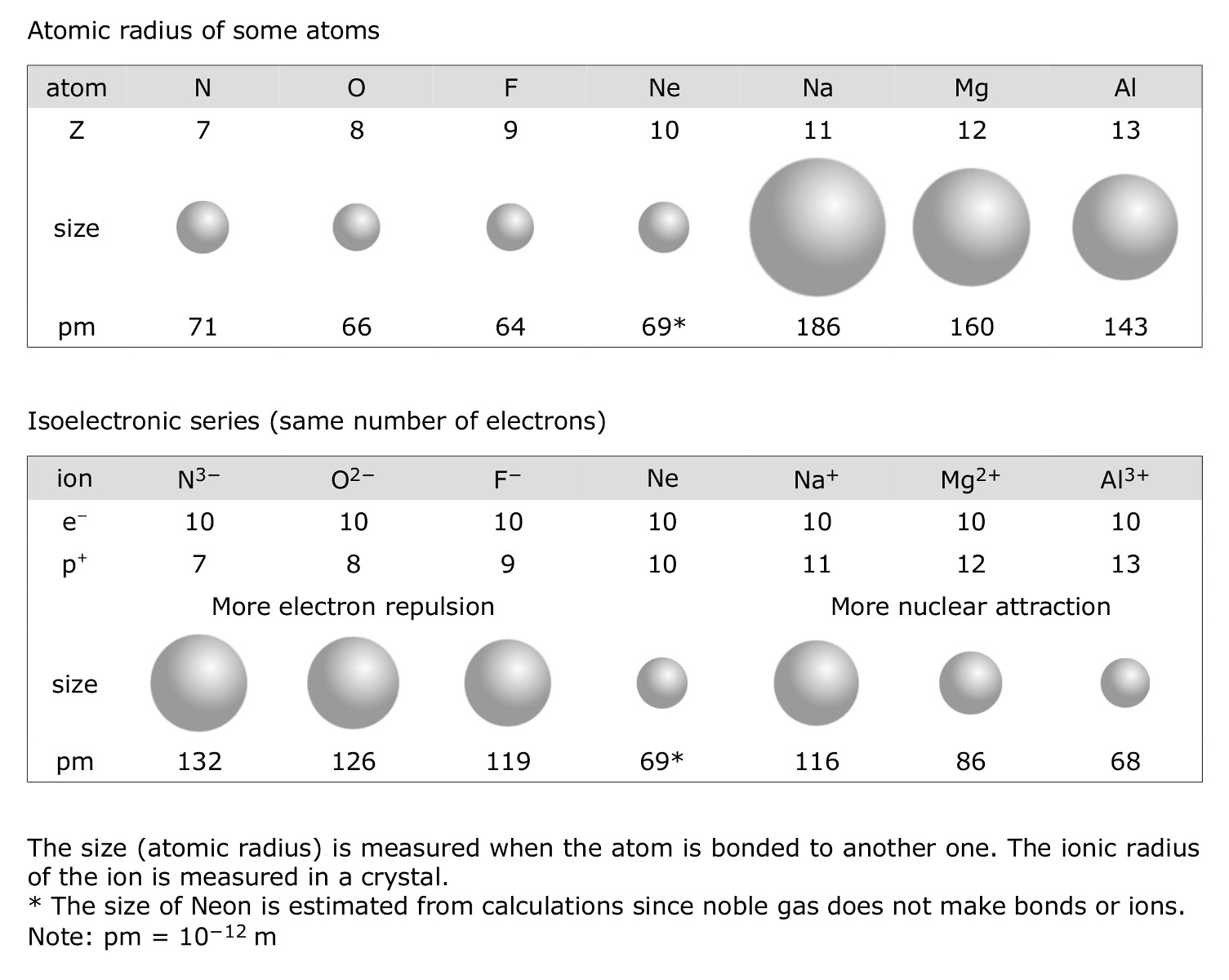
The ionization energy
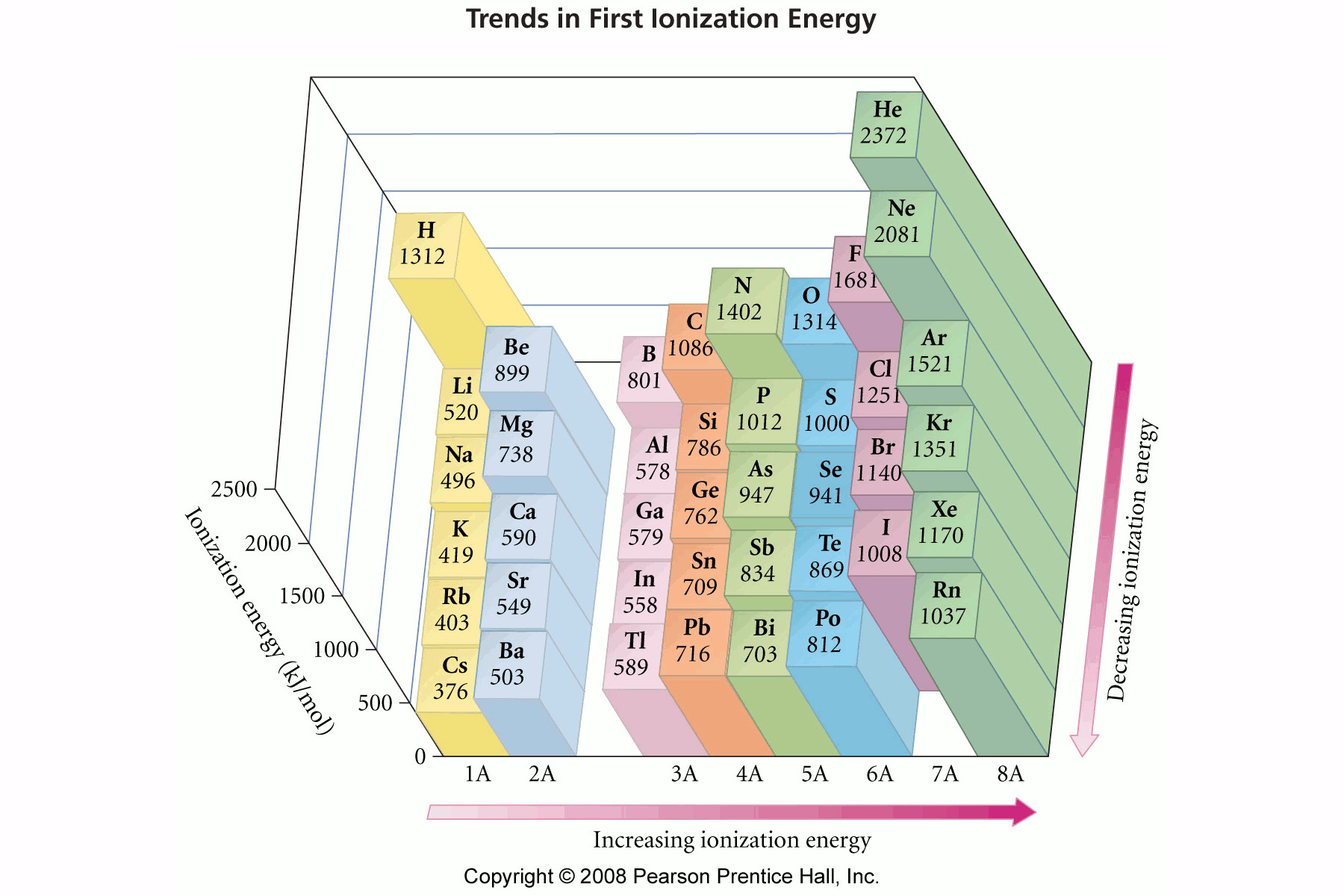
Also, the outermost electrons are also shielded by the electrons closest to the nucleus (in the lower orbitals).
The consequence is that metals (to the left of the periodic table) are easier to ionize than non-metal.
Smaller atoms (top right of the periodic table) are therefore more difficult to ionize.
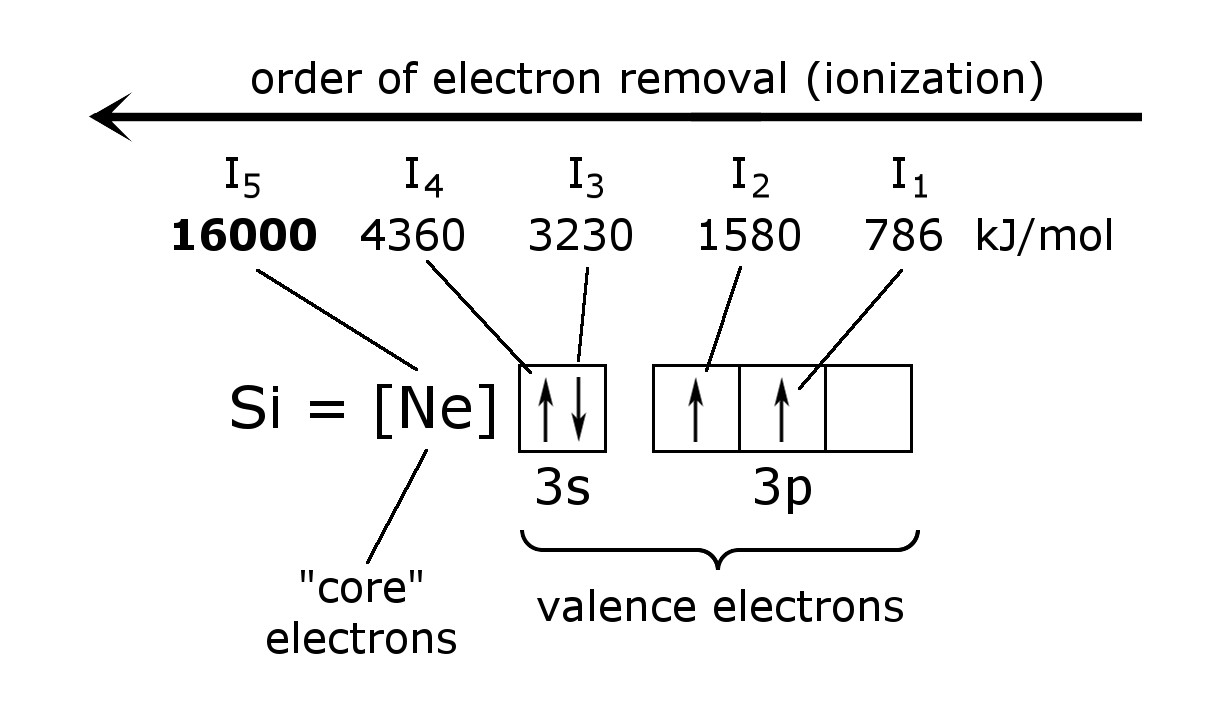
When several electrons are removed from an atom, the required ionization energy always increases for each additional electron removed.
I1 < I2 < I3 ...
The amount of successive ionization energy is related to the orbital from which the electron is removed.
The first 4 electrons removed have an ionization energy ranging from 786 kJ/mol to 4360 kJ/mol. However, an enormous amount of energy is required to remove the 5th electron. This one, in the case of silicon is a central electron (or core electron). These are never available for bonding.
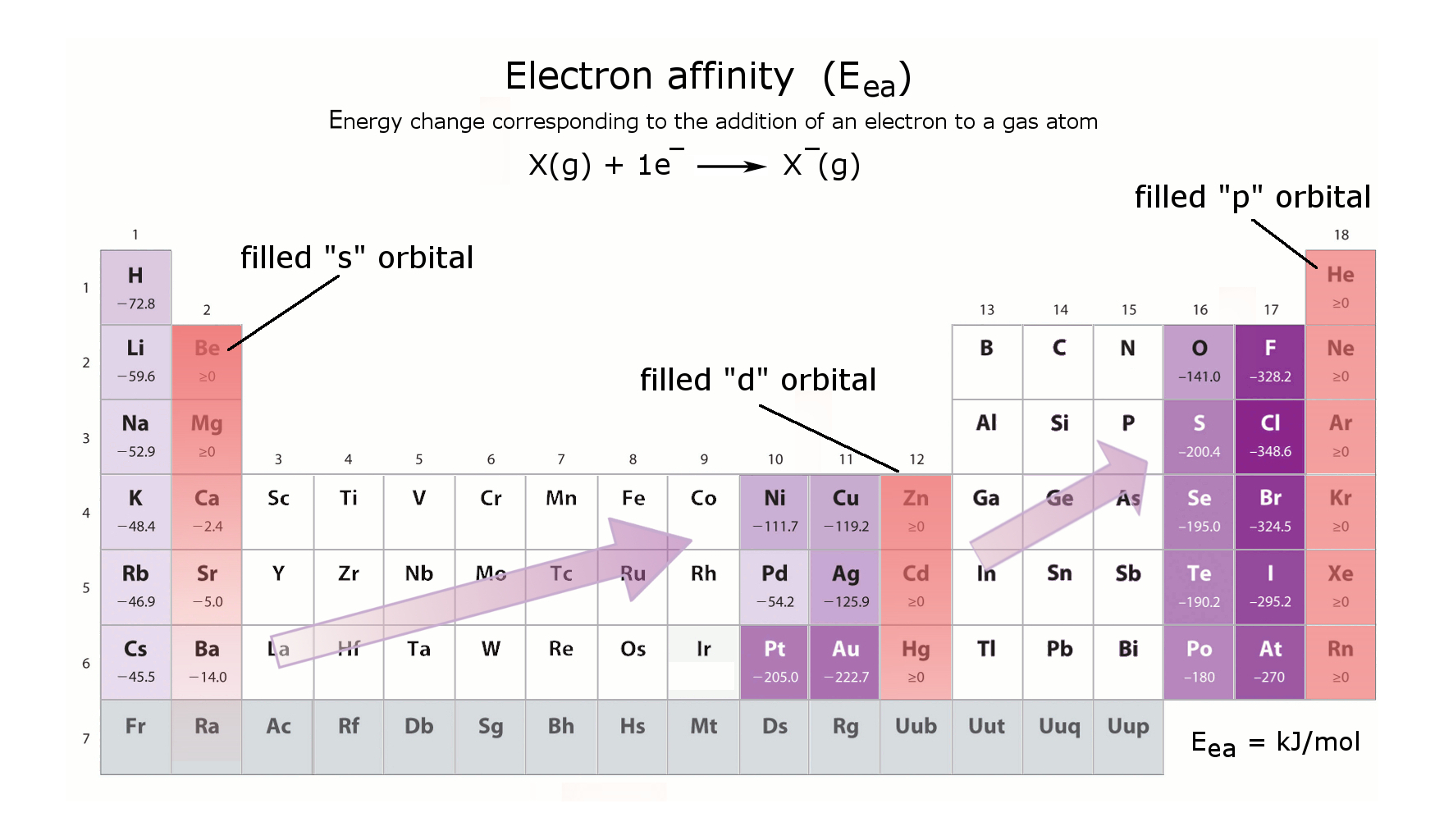
Electron affinities (Eea)
An atom can take an additional electron when an orbital is available.
Example: Li: 1s22s1. An additional electron can be placed in the 2s1 orbital to become 2s2
However, for Beryllium "Be", the 2s2 orbital is filled = no electron affinity.
Any atom that requires one extra electron to complete its s, p, d orbital has a high electron affinity (eg. K, Cu, Cl)
Any atom with a filled orbital has no electron affinity (eg. Mg, Zn, Xe)
Electronegativity
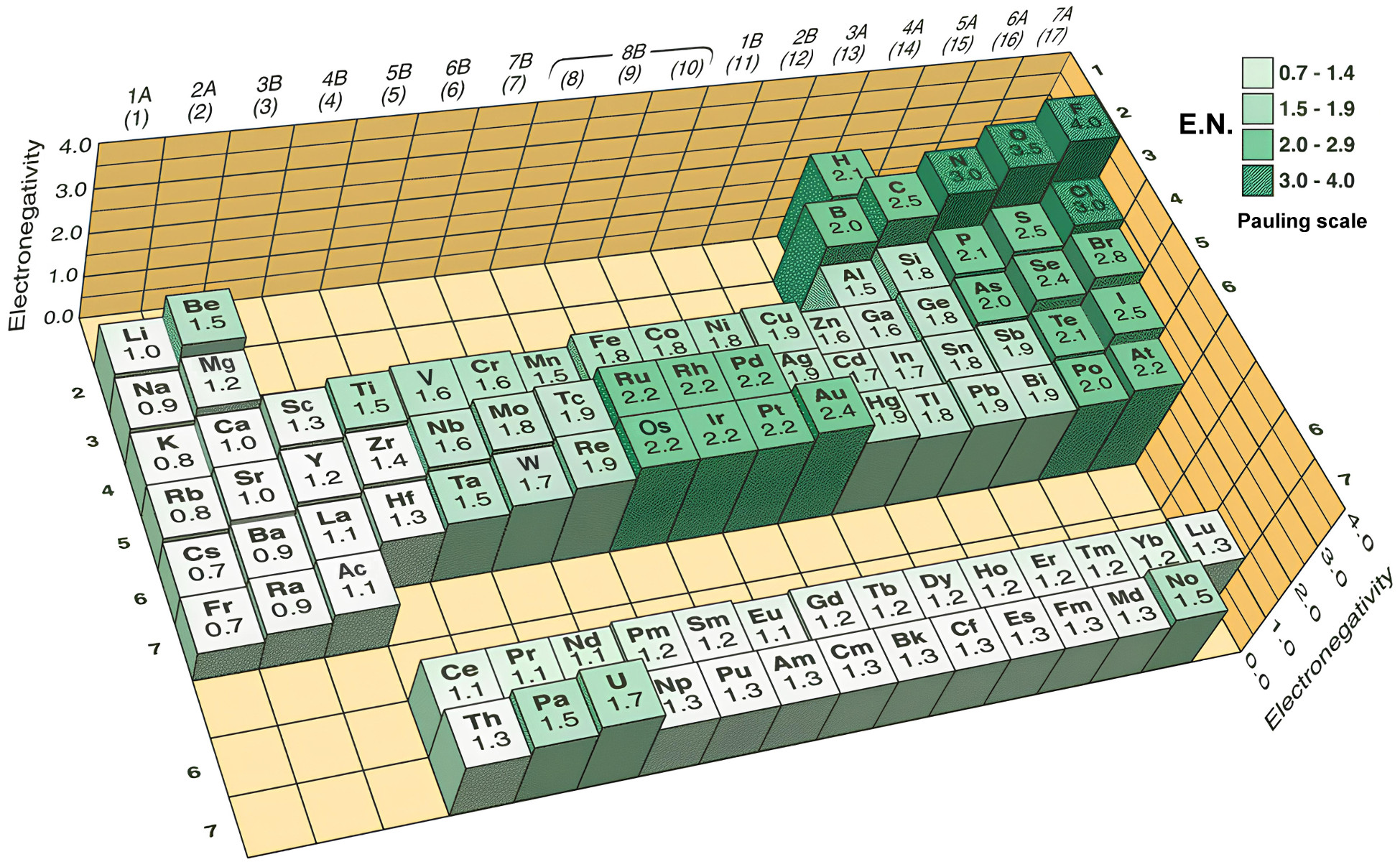
Often, the smallest atoms (top right of the periodic table) are also the most electronegative.
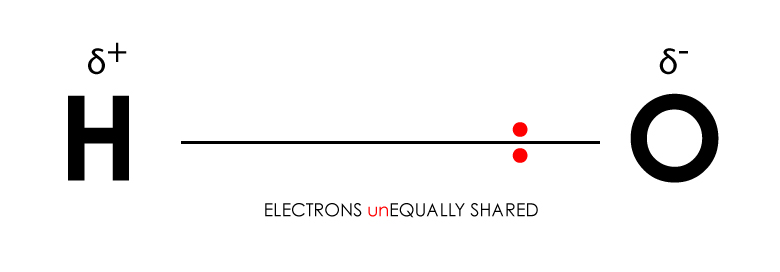
Representation of an unequal distribution of the electron between H-O in a covalent bond (O more electronegative than H)
Periodic trends explained (video)
You should be able to do the following problems Zumdahl 11th ed: Chap 7. # 119, 123, 133, 135.